P.E.T® Delivery
Technology
Technology
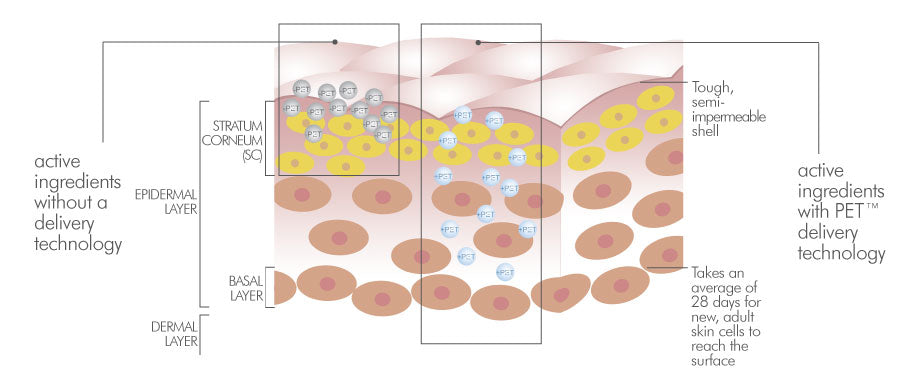
A patented drug-delivery system from SESHA Skin Therapy (Conrex Pharmaceuticals) that enhances ingredient absorption into deeper layers of skin without damaging the barrier. Safe, effective, and clinically backed.
PET™ is a drug delivery technology that works by solubilizing the active ingredient(s) within the polymeric matrices and temporarily modifying the permeability of the skin, thus enabling large molecules to pass between and through the skin cells of the stratum corneum, stratum lucidum, stratum granulosum and ultimately reaching the deeper layers of the skin (stratum spinosum, stratum basale) .
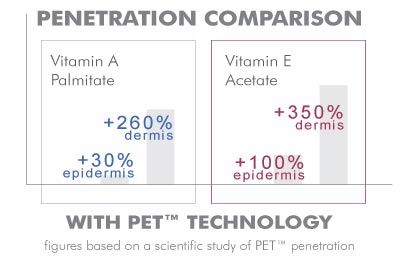
Permeation evaluation of two topical preparations, one with Vitamin A palmitate, and the second with Vitamin E acetate . Both were combined with SESHA SKIN THERAPY’s PET™ delivery system. Permeation results were compared against placebo creams and were measured using Franz diffusion cells .
Two cream preparations, each with and without PET™, applied topically to test subject.
PET™ increased the retention of Vitamin A in the epidermis by 30% and in the dermis by 260%. PET™ demonstrated an increase of Vitamin E in the epidermis by 100% and in the dermis by 350%.
PET™ was evaluated by pre-clinical studies to test the efficacy of PET™ in drug formulations. Listed below are three of the pre-clinical studies done by our scientists to document the increased efficacy of drug permeation.
Evaluation of the in vitro percutaneous (skin) permeation of 1% Diclofenac Acid gel prepared with PET™ delivery system. Permeation results are compared to an experimental control (1% Diclofenac Acid containing no PET™) and a commercially available preparation of 1% Diclofenac Acid (Voltaren® Emulgel).
Evaluation of the in vitro percutaneous permeation enhancement of 1% clotrimazole preparation containing 4% PET™. Permeation results are compared to an experimental control (1% clotrimazole gel without PET™) and, a commercially available preparation of 1% clotrimazole (Lotrimin® AF topical solution).
Evaluation of the in vitro percutaneous permeation enhancement of a testosterone gel containing 8% PET™. Permeation results are compared to an experimental control (testosterone gel without PET™).
Conrex Pharmaceutical clinical studies were performed to test the efficacy of PET™ in developing the SESHA SKIN THERAPY skincare line. These studies show SESHA SKIN THERAPY is based on scientific proof of PET™ effectiveness in delivering pharmaceutical grade nutrients, cutting-edge antioxidants, superior quality botanicals, peptides, and other powerfully active ingredients to the deepest layers of the skin.
Evaluation of the efficacy of SESHA SKIN THERAPY A.C.E. Cream with PET™ delivery system. Changes in the skin’s epidermal density, thickness, color and wrinkles depth were monitored using ballistometry, ultrasound B-mode scans, photographs, clinical assessments, silastic castings, and skin sample biopsies.
Comparison of epidermal cell proliferation produced by SESHA SKIN THERAPY A.C.E. Cream containing PET™ nutrient delivery system with Retin-A Cream (0.025%) and Oil of Olay Original Beauty Fluid.
Research the effectiveness of Super C Serum and A.C.E. PLUS Cream in reducing fine lines and improving skin texture.
Clinical study shows PET™ could be an effective vehicle to deliver RPM in to the dermis in conjunction with laser therapy to inhibit Port Wine Staine (PWS).
The advantage of topical application is that RPM could be delivered to the dermis while avoiding significant systemic drug absorption and associated side effects [41] . Two different topical RPM formulae were tested in this study. In both formulae, a skin penetration enhancer (PET™) was used. The results indicate that RPM can be effectively delivered through the stratum corneum using this enhancer.
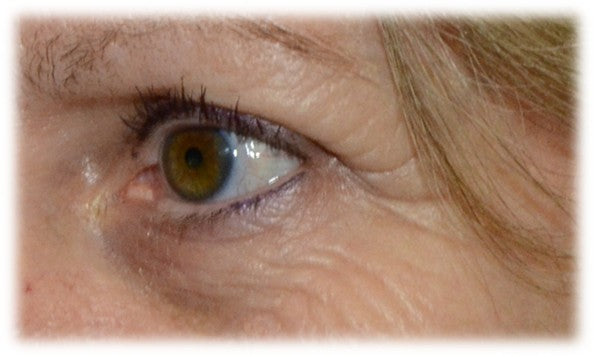
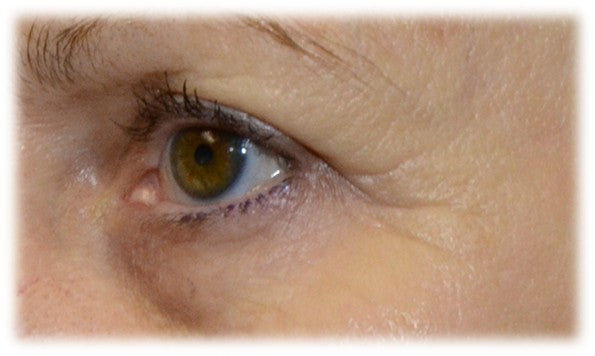
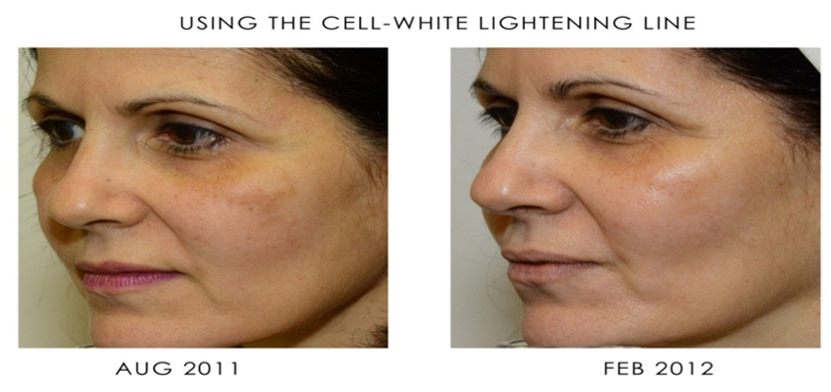
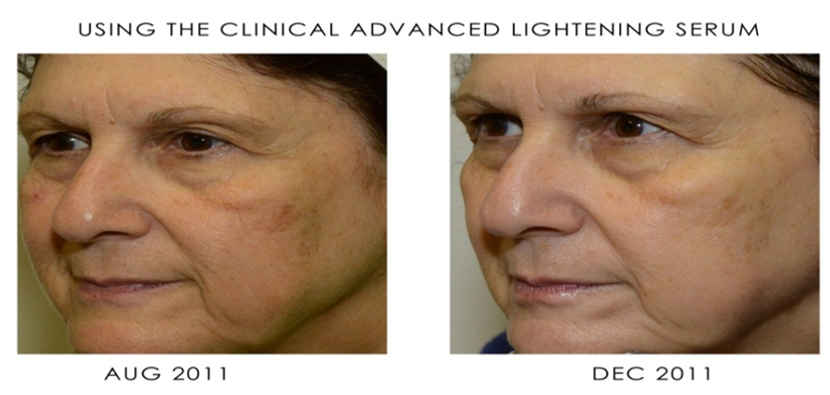
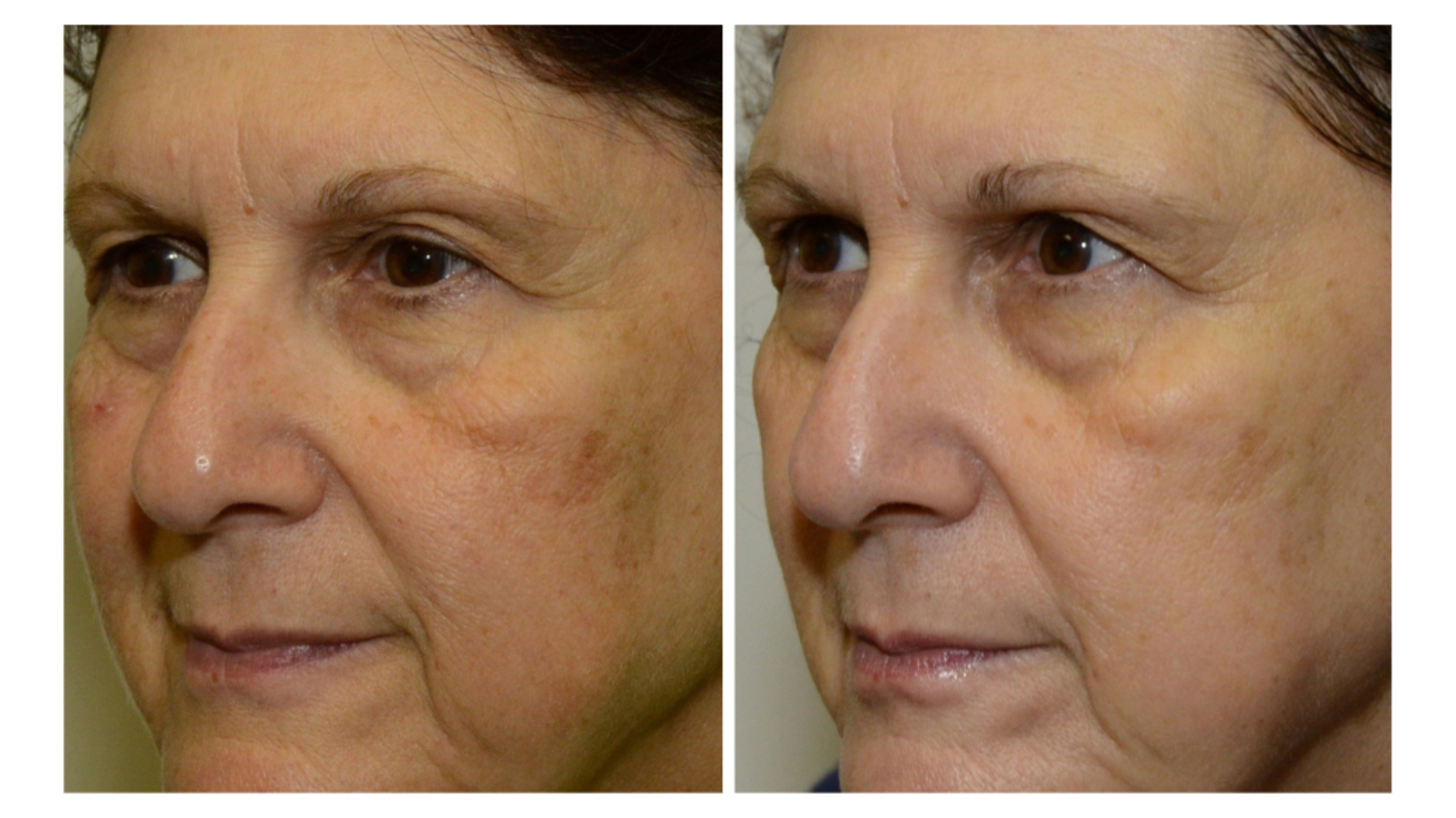
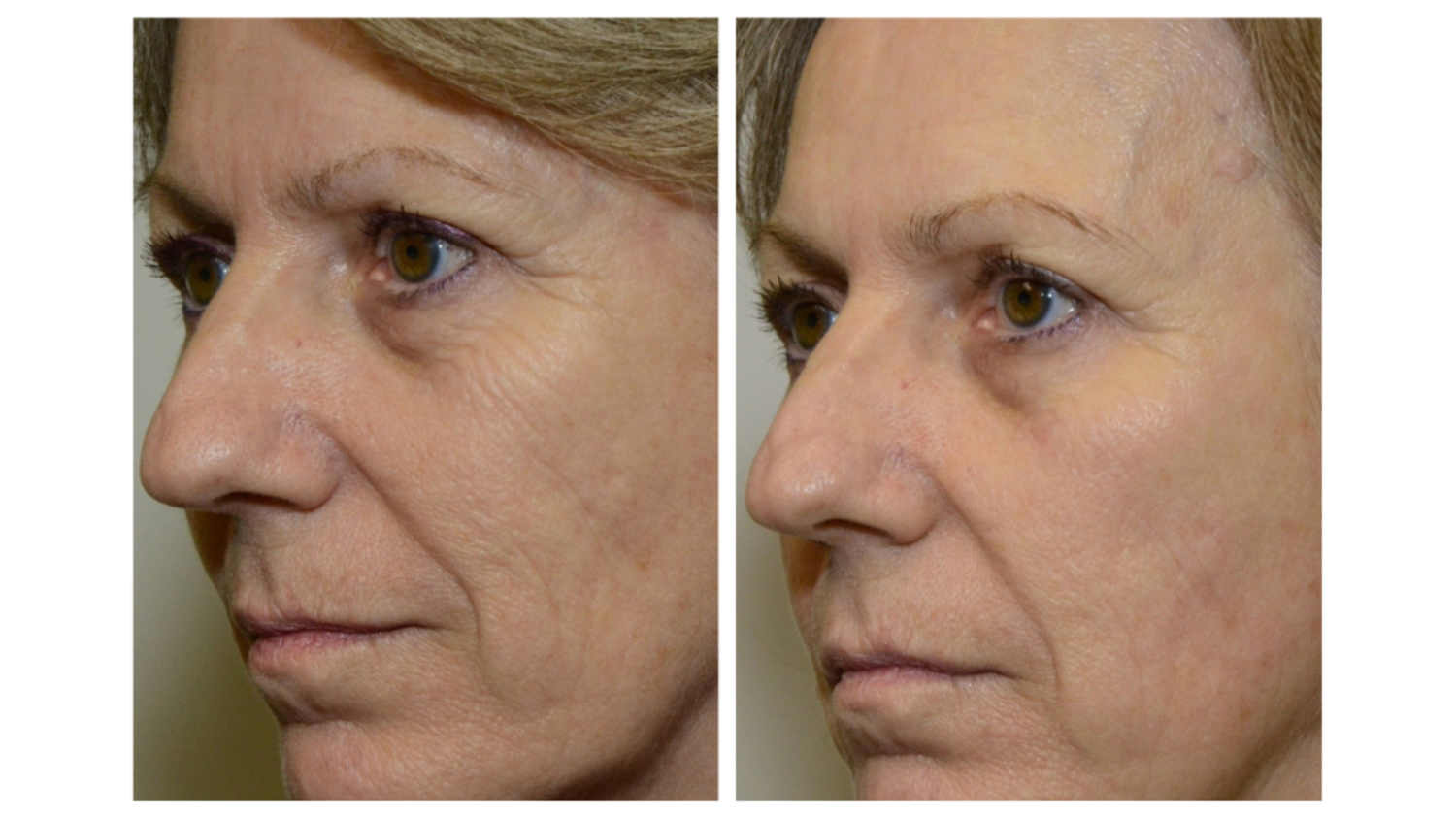
Sesha Skin Therapy(Conrex Pharmaceutical) utilizes scientific books and publications to disseminate research findings and increase understanding of its patented technologies.
Create a professional account login today to view our pre-recorded MesoBotanica video demo and informational guide.